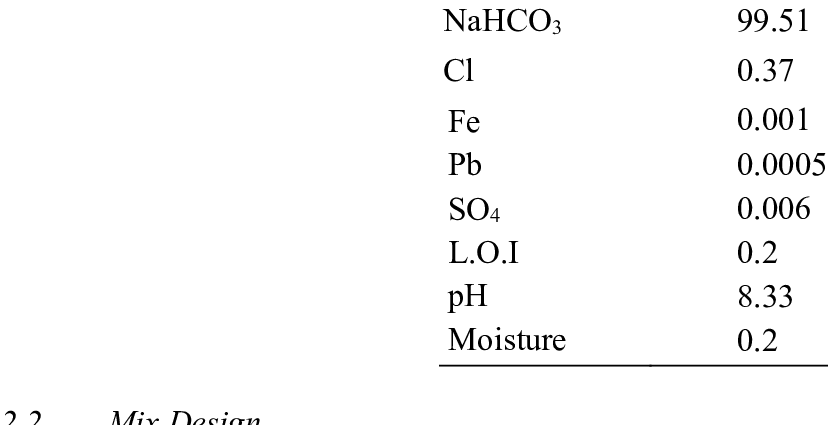
ọdịnaya
Nyocha nke bicarbonate
Nkọwa nke bicarbonates
The ion bicarbonates (HC03-) dị n'ime ọbara: ha na-ekere òkè dị ukwuu na usoro pH. Ha bụ "ihe nchekwa" nke anụ ahụ.
Ya mere, itinye uche ha n'ọbara na-adaba na pH. Ọ bụ tumadi akụrụ na-achịkwa ntinye nke bicarbonates ọbara, na-akwalite njide ma ọ bụ excretion ha.
Iji dozie pH, ion bicarbonate HCO3- na-ejikọta ya na H ion+ inye mmiri na CO2. Ọnụ ego nke en CO2 N'ime ọbara akwara (PA CO2), ma ọ bụ capnia, ma ọ bụ nrụgide akụkụ nke CO2 na-agbaze n'ime ọbara akwara, bụkwa ihe na-egosi nguzozi acid-base. A na-atụ ya n'oge nyocha nke gas ọbara.
Ion bicarbonate bụ isi: mgbe itinye uche ha na-abawanye, pH na-abawanye. N'aka nke ọzọ, mgbe itinye uche ha na-ebelata, pH na-aghọ acidic.
N'ime onye ahụ siri ike, pH ọbara kwụsiri ike: 7,40 ± 0,02. Ọ gaghị ada n'okpuru 6,6 ma ọ bụ bilie n'elu 7,7, nke na-adabaghị na ndụ.
Kedu ihe kpatara nyocha nke bicarbonate?
Usoro nke ion bicarbonate na-eme ka o kwe omume iji nyochaa nguzozi acid-base nke ọbara. A na-eme ya n'otu oge ahụ dị ka nyocha nke gas ọbara, mgbe dọkịta na-enyo enyo na ọnụnọ nke enweghị acid-base (acidosis ma ọ bụ alkalosis). Nke a nwere ike ịbụ ikpe na ọnụnọ ụfọdụ mgbaàmà, dịka:
- ọnọdụ nsụhọ gbanwere
- hypotension, obere mmepụta obi
- nsogbu iku ume (hypo- ma ọ bụ hyperventilation).
- Ma ọ bụ n'ọnọdụ ndị na-adịchaghị njọ dị ka mgbari nri na-adịghị mma ma ọ bụ mfu urinary ma ọ bụ ọgba aghara electrolyte.
Nyochaa nke bicarbonates
Nnwale ọbara ahụ nwere ihe nlele nke ọbara venous, na-emekarị n'ogige nke ikpere. Ọ dịghị nkwadebe dị mkpa.
Kedu ihe anyị nwere ike ịtụ anya site na nyocha nke bicarbonates?
Nyocha ahụ na-eme ka o kwe omume ịchọpụta ọnụnọ nke acidosis ma ọ bụ a alkalosis. Ntụle pH ga-enye gị ohere ịhụ ma enwere hyperacidemia (akọwapụtara dị ka pH uru n'okpuru 7,35) ma ọ bụ hyperalcalemia (pH uru karịa 7,45).
Ntụle nke ion bicarbonate na PaCO2 mgbe ahụ na-enye ohere iji chọpụta ma ọ bụrụ na nsogbu ahụ sitere na metabolic si malite (ọdịdị nke bicarbonates) ma ọ bụ iku ume (adịghị mma nke PaCO).2). Nkịtị maka bicarbonates dị n'etiti 22 na 27 mmol / l (millimoles kwa liter).
Mbelata mkpokọta ion bicarbonate n'okpuru ụkpụrụ nkịtị na-ebute ọrịa metabolic acidosis. Acidosis jikọtara ya na oke H + ion. N'ihe banyere acidosis nke metabolic, a ga-ebelata mkpokọta ion bicarbonate (pH <7,35). N'ime acidosis iku ume, ọ bụ mmụba na nrụgide akụkụ nke CO2 nke ga-abụ maka mmụba nke ion H +.
Metabolic acidosis nwere ike ịbụ n'ihi, n'etiti ihe ndị ọzọ, na mfu nke bicarbonates na-adịghị mma n'ihi afọ ọsịsa ma ọ bụ infusion saline physiological.
N'aka nke ọzọ, mmụba na ntinye nke ion carbonate na-eduga na a metabolic alkalosis (pH> 7,45). Ọ nwere ike ime ma ọ bụrụ na oke nchịkwa nke bicarbonates, ọgbụgbọ siri ike ma ọ bụ enweghị potassium (diuretics, afọ ọsịsa, vomiting). Hyperaldosteronism nwekwara ike itinye aka (hypersecretion nke aldosterone).
Alkalosis iku ume, maka akụkụ ya, kwekọrọ na mbelata dịpụrụ adịpụ na nrụgide akụkụ nke CO2.
Gụọ kwa: Ihe niile gbasara hypotension |